- Home
- Billionaires
- Investing Newsletters
- 193CC 1000
- Article Layout 2
- Article Layout 3
- Article Layout 4
- Article Layout 5
- Article Layout 6
- Article Layout 7
- Article Layout 8
- Article Layout 9
- Article Layout 10
- Article Layout 11
- Article Layout 12
- Article Layout 13
- Article Layout 14
- Article Sidebar
- Post Format
- pages
- Archive Layouts
- Post Gallery
- Post Video Background
- Post Review
- Sponsored Post
- Leadership
- Business
- Money
- Small Business
- Innovation
- Shop
Recent Posts
Eli Lilly Warns of Lawsuits Over Counterfeit Weight-Loss Drugs
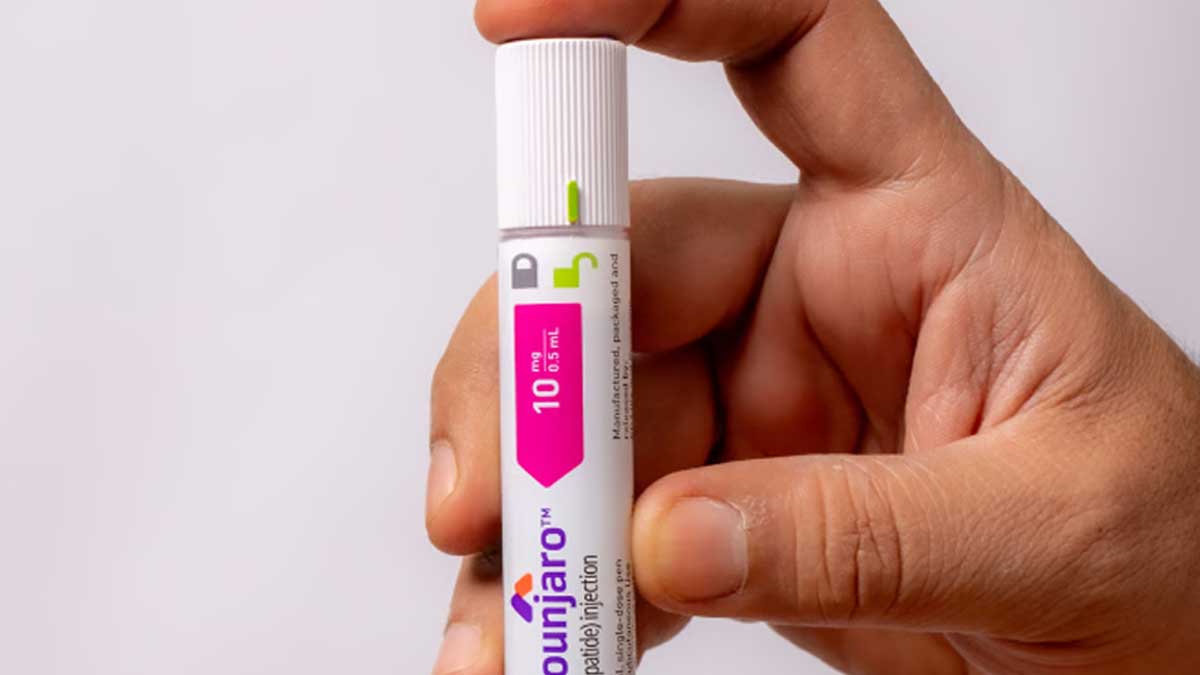
Several medical spas and wellness centers face potential lawsuits from Eli Lilly for allegedly selling compounded and counterfeit versions of popular weight loss and diabetes drugs Mounjaro and Zepbound. This issue, also highlighted by Ozempic maker Novo Nordisk, has prompted health organizations to warn of serious side effects such as infections from these counterfeit products.
In an open letter on Thursday, Eli Lilly, the maker of Mounjaro and Zepbound, cautioned against using drugs labeled “research purposes only” or “not for human consumption.” The company noted that federal regulators have not approved oral versions of these drugs, although pills have appeared online.
Eli Lilly claims some wellness centers and websites are selling unauthorized versions of these drugs, made with unapproved chemicals and marketed as generics, including compounded versions with altered ingredients. Lilly clarified that it does not sell generic versions of its drugs.
The company warned that counterfeit versions of its products could cause serious harm due to incorrect dosages, wrong medications, or mixed medications. These fake products have been found to contain bacteria, high impurity levels, and different chemicals from Lilly’s genuine drugs, raising safety, efficacy, and sterility concerns.
The FDA also warned against counterfeit semaglutide—the generic name for Ozempic and Wegovy—sold by unauthorized online retailers due to potential adverse events like infection and abdominal pain. As of March 31, the FDA received over 100 adverse event reports from counterfeit tirzepatide and semaglutide products since 2020, including life-threatening cases, 19 hospitalizations, and at least two deaths.
To spot fake GLP-1 drugs, Lilly advises checking for a colorless appearance—some fake versions have a pink hue. Any product labeled as generic tirzepatide or semaglutide is fake, as neither Lilly nor Novo Nordisk sells generic versions. Incorrect dosages, grammatical errors on packaging, lack of tamper-resistant perforation, and mismatched batch numbers are also signs of counterfeit drugs.
The National Association of Boards of Pharmacy reported that illegal actors exploit high demand and short supply to sell substandard and falsified versions of these products, endangering patients. Due to high demand, Wegovy, Ozempic, Zepbound, and Mounjaro have been in shortage for months. Lilly warned that fake versions of its products are also being sold online and on social media, where the company does not sell genuine Zepbound and Mounjaro.
In its open letter, Lilly stated it is seeking legal action against medspas, wellness centers, and other clinics selling unapproved compounded and counterfeit versions of its products. Lilly alleges these clinics mislead customers by referring to the fake products as Mounjaro and Zepbound, using Lilly’s clinical trial results to market them, and deceptively using FDA approval to sell the fake products. Previously, Lilly settled a lawsuit with South Carolina-based Totality Medispa, which agreed to pay an undisclosed amount and comply with U.S. federal law in distributing compounded tirzepatide products. Novo Nordisk also recently filed lawsuits against nine wellness clinics for selling compounded versions of Ozempic and Wegovy, which contained up to 24% impure chemicals, seeking to halt their sales and claiming up to $75,000 in compensation.
Recent Posts
Categories
- 193cc Digital Assets2
- 5G1
- Aerospace & Defense46
- AI37
- Arts3
- Banking & Insurance11
- Big Data3
- Billionaires449
- Boats & Planes1
- Business328
- Careers13
- Cars & Bikes76
- CEO Network1
- CFO Network17
- CHRO Network1
- CIO Network1
- Cloud10
- CMO Network18
- Commercial Real Estate7
- Consultant1
- Consumer Tech180
- CxO1
- Cybersecurity68
- Dining1
- Diversity, Equity & Inclusion4
- Education7
- Energy8
- Enterprise Tech29
- Events11
- Fintech1
- Food & Drink2
- Franchises1
- Freelance1
- Future Of Work2
- Games141
- GIG1
- Healthcare78
- Hollywood & Entertainment186
- Houses1
- Innovation42
- Investing2
- Investing Newsletters4
- Leadership65
- Lifestyle11
- Manufacturing1
- Markets20
- Media193
- Mobile phone1
- Money13
- Personal Finance2
- Policy567
- Real Estate1
- Research6
- Retail1
- Retirement1
- Small Business1
- SportsMoney33
- Style & Beauty1
- Success Income1
- Taxes2
- Travel10
- Uncategorized8
- Vices1
- Watches & Jewelry2
- world's billionaires418
Related Articles
What Healthcare Can Learn from Nvidia’s Success
The tech industry is undergoing a seismic transformation, with two of its...
By 193cc Agency CouncilDecember 16, 2024Salmonella Triggers Recalls of Costco Eggs and Cucumbers
The recent salmonella outbreak has prompted the recall of two major food...
By 193cc Agency CouncilNovember 30, 2024Bird Flu Found in Raw Milk in California, Recall Issued
California health authorities have confirmed the presence of the bird flu virus...
By 193cc Agency CouncilNovember 25, 2024UniDoc Health Launches Mobile ‘Health Cube’ for Remote Care
UniDoc Health, a Vancouver-based company, is revolutionizing healthcare accessibility with the launch...
By 193cc Agency CouncilNovember 23, 2024















Leave a comment